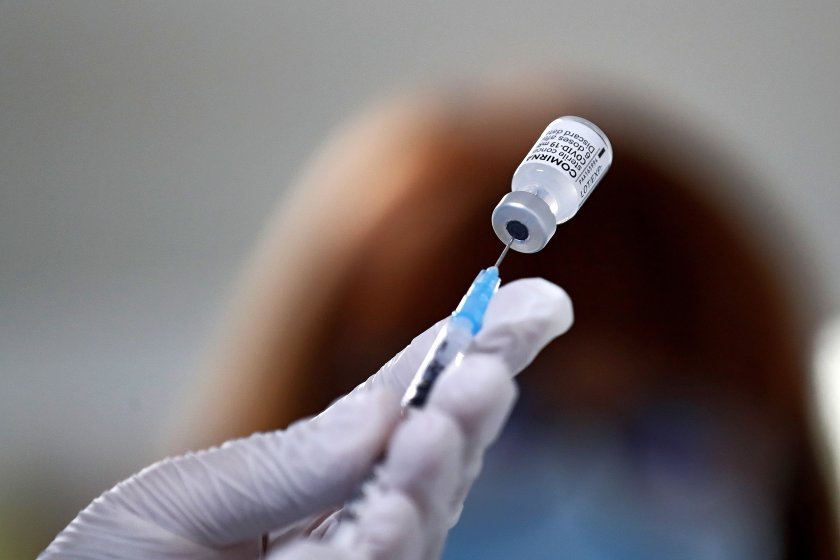
The European Medicines Agency (EMA) has approved the application of a third dose of the BioNTech-Pfizer and Moderna vaccines against Covid-19 for persons aged 18 years and over.
EMA says that the third dose may be given to people at least 6 months after their second dose.
Here is the full text of the announcement:
EMA’s human medicines committee (CHMP) has concluded that an extra dose of the COVID-19 vaccines Comirnaty (BioNTech/Pfizer) and Spikevax (Moderna) may be given to people with severely weakened immune systems, at least 28 days after their second dose.
The recommendation comes after studies showed that an extra dose of these vaccines increased the ability to produce antibodies against the virus that causes COVID-19 in organ transplant patients with weakened immune systems.
Although there is no direct evidence that the ability to produce antibodies in these patients protected against COVID-19, it is expected that the extra dose would increase protection at least in some patients. EMA will continue monitoring any data that emerges on its effectiveness.
The product information of both vaccines will be updated to include this recommendation.
Booster doses
It is important to distinguish between the extra dose for people with weakened immune systems and booster doses for people with normal immune systems.
For the latter, the CHMP has evaluated data for Comirnaty showing a rise in antibody levels when a booster dose is given approximately 6 months after the second dose in people from 18 to 55 years old. On the basis of this data, the Committee concluded that booster doses may be considered at least 6 months after the second dose for people aged 18 years and older.
At national level, public health bodies may issue official recommendations on the use of booster doses, taking into account emerging effectiveness data and the limited safety data. The risk of inflammatory heart conditions or other very rare side effects after a booster is not known and is being carefully monitored. As for all medicines, EMA will continue to look at all data on the safety and effectiveness of the vaccine.
More information about the booster recommendations for Comirnaty will be available in the updated product information.
The Committee is currently evaluating data to support a booster dose for Spikevax. EMA will communicate the outcome when the evaluation is complete.
National immunisation campaigns
The implementation of vaccination campaigns in the EU remains the prerogative of the national immunisation technical advisory groups (NITAGs) guiding the vaccination campaigns in each EU Member State. These bodies are best placed to take into account the local conditions, including the spread of the virus (especially any variants of concern), the availability of vaccines and the capacities of national health systems.
EMA will continue working closely with national authorities and the European Centre for Disease Prevention and Control (ECDC) to evaluate available data and provide recommendations to protect the public during the ongoing pandemic.








 Чуй новините
Чуй новините Подкаст
Подкаст